The Problem
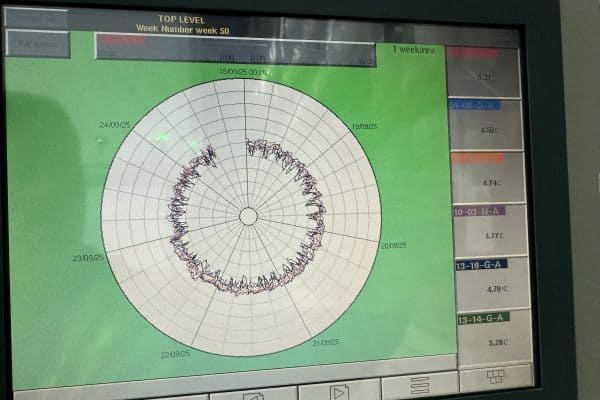
A long-standing pharmaceutical customer in North Wales relied on a critical coldroom facility to protect over £40 million worth of stock. The products must be stored between 2°C and 8°C at all times. Any deviation outside this range leads to quarantined stock, regulatory paperwork, and potential product loss.
The existing refrigeration system, installed by ICE in 2005, had performed faultlessly for two decades. However, challenges were mounting:
- Ageing infrastructure: Equipment was becoming obsolete, with spare parts increasingly unavailable.
- Regulatory pressure: The system ran on R407C, a refrigerant with a Global Warming Potential (GWP) of 1774, far above the thresholds being phased out under F-Gas legislation.
- Future risk: By 2030, all new refrigeration systems must use refrigerants with a GWP <150. Businesses relying on high-GWP refrigerants face not only compliance risks but also escalating costs and supply restrictions.
The customer needed a modern, compliant, and future-proof solution, delivered without interrupting operations or putting stock at risk.
The Solution
ICE designed and installed a cutting-edge refrigeration system built around R454C (A2L) refrigerant, with a GWP of just 146. This ensures the installation is fully compliant with current F-Gas rules and future-ready well beyond 2030.
Why A2L?
- Ultra-low GWP: Over 90% lower than the outgoing R407C, dramatically reducing environmental impact.
- Energy efficiency: Comparable to traditional HFCs, meaning no compromise on performance.
- Safe and practical: Classified as “mildly flammable” (A2L), R454C offers low toxicity and requires only minor adjustments to established installation practices.
- Future-proof: Protects against refrigerant bans, price spikes, and compliance risks tied to high-GWP gases.
Equipment & Engineering Design
To deliver a reliable, high-performance system, ICE deployed proven, industry-leading equipment:
- Main Area: Served by Friga Bohn packaged DUO condensing units, each with twin compressors, integral condensers, filter driers, and safety controls – providing resilience and efficiency.
- Upper & Lower Mezzanines: Served by Danfoss Optyma Plus condensing units, featuring advanced scroll compressors and integrated fault diagnostics for easy servicing and reliability.
- Coolers: High-efficiency Kelvion evaporator coolers with upgraded fans to ensure optimal airflow, carefully selected to overcome layout constraints caused by the legacy system.
Working with QA & Documentation
Because of the critical nature of pharmaceutical storage, ICE worked hand-in-hand with the customer’s Quality Assurance department from start to finish. This included:
- Multiple QA review meetings to agree installation methodology.
- Detailed risk assessments and method statements, ensuring product integrity throughout.
- Full changeover documentation to demonstrate compliance and continuity.
- Comprehensive O&M manuals, including drawings, specifications, and maintenance guidelines, handed over at project completion.
- Delivery of all required PED Category 2 certification.
This thorough, documented approach gave the customer absolute confidence that their regulatory obligations and stock protection requirements were fully met.
Built-In Redundancy
Unlike the outgoing system that relied on two run/standby chillers, the new installation consists of 15 fully independent refrigeration systems. This means:
- The room can maintain temperature even if one or more systems are offline.
- Redundancy is built into the design, with no single point of failure.
- Maintenance and servicing can be carried out without interrupting operations.
For a facility storing tens of millions of pounds worth of stock, this new design provides unparalleled resilience and peace of mind.
The Outcome
The coldroom was successfully handed over in August 2025:
- Compliance secured: The system now fully complies with the F-Gas phase down, eliminating long-term refrigerant risk.
- Temperature integrity maintained: The room stayed within the strict 2–8°C range throughout installation, avoiding stock quarantines or losses.
- Operational resilience: With 15 independent systems, the facility now benefits from maximum redundancy, removing reliance on run/standby pairs.
- Seamless QA approval: With ICE’s risk assessments, O&M manuals, and PED certification in place, QA sign-off was smooth and stress-free.
- Future-proof performance: The customer now has a sustainable, reliable system designed to last 20+ years, ensuring security for both regulatory compliance and product safety.